Heat from electricity
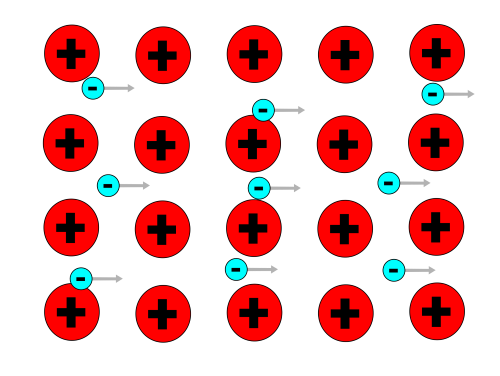
What exactly happens? There is a model that describes electric current: atoms, which make up all matter, are electrically neutral. But whole atoms do not have determined places in a metalic conductor. Instead there is a lattice of atomic cores, through which the corresponding electrons can move freely.
Whenever an electric current flows, it means that electrons move in between the cores. We can imagine the interaction between the electrons and atomic cores during this movement like the electrons bumping into the cores. The electrons emit a part of their kinetic energy to the atomic cores, which makes the cores and the whole lattice vibrate. Because of this, the lattice, that is the whole metal object, has an inner vibration energy, which we call heat energy. The higher the temperature of an object, the greater the lattice vibration.
The interaction between electrons and atomic cores transforms electrical energy into heat energy.
Move the cursor over the image to increase the temperature.
The electrons have lost some of their kinetic energy and the lattice vibrates more.
