Structure of atoms
All matter is made out of atoms, no matter if it is solid, liquid or gas. All features of objects can be traced back to the structure and interaction of their atoms.
Although atoms are so important, they are very small. If we tried to make a chain of atoms that is one millimeter long, we would require 10 million atoms.
The structure of atoms is particularly important. They consist of a core and a shell. The atomic core consists of positively charged protons and neutrally charged neutrons. The atomic shell consists of electrons, which are charged negatively.
The number of protons determins what kind of element (eg. iron, gold or oxygen) the atom builds. For example, the following image depicts the structure of an aluminium atom.
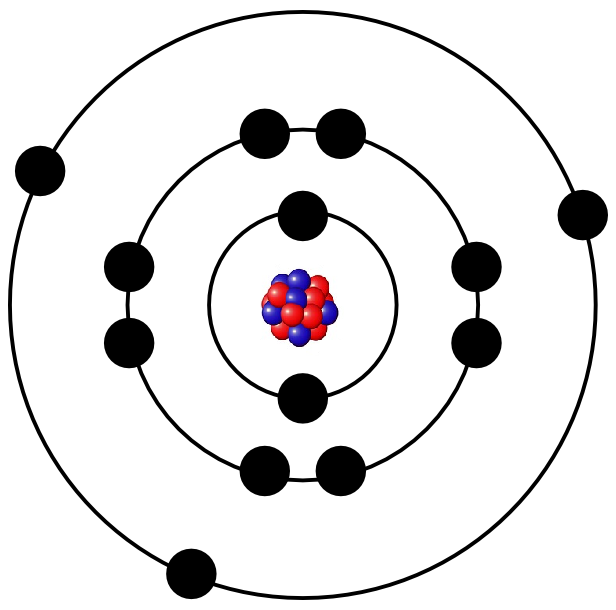
Because protons and neutrons are far heavier than electrons, most of an atoms mass comes from its core. However, the electrons make up the atomic shell and therefore a big part of the atomic volume. If an atomic core was the size of a pea, its shell would be as big as a whole stadium with its stands.
In the study of electricity, it is substantial that the charge of an object is determined by its atoms. Charge transport usually happens with negative charges, that is electrons. They can move easier because they are not bound to the core.
