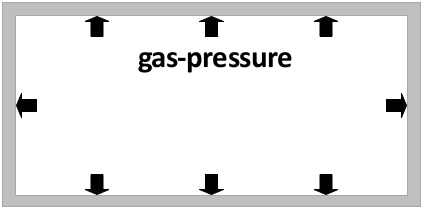
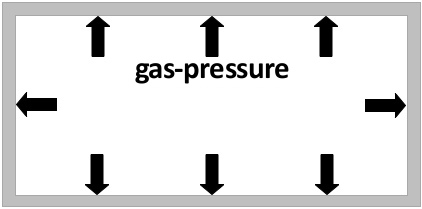
Gas pressure
If you have a sealed amount of gas, the pressure of the pressure of the gas inside the container is proportional to the temperature of the gas. (The higher the temperature of the gas, the higher the pressure.)
The temperature of the gas is a measurement for the average speed of the gas particles. The higher the temperature, the higher the average speed of the gas particles. The faster the gas particles clash to the container walls the higher the pressure inside of the container increases.
In the left picture the short arrows symbolise the gas pressure with a low temperature. The longer arrows in the right picture symbolise the higher pressure that arises because the gas was heated up.