Gas pressure
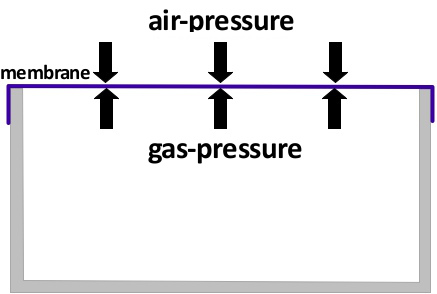
The picture above shows a container that is closed with a membrane that is easy to extend. The pressure of the gas inside of the container is equal to the outside air-pressure. Also, the temperatures inside and outside of the container are the same.
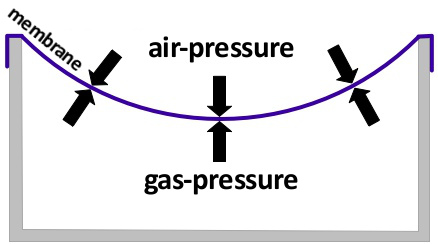
In the picture above the container was cooled down. The pressure of a sealed amount of gas is lowered when the temperature is lowered aswell. Because the membrane is extendable the gas inside of the container is compressed by the outside air-pressure. That has the effect that the pressure inside increases again. The membrane therefore allows the container to have the same pressure as the air outside.
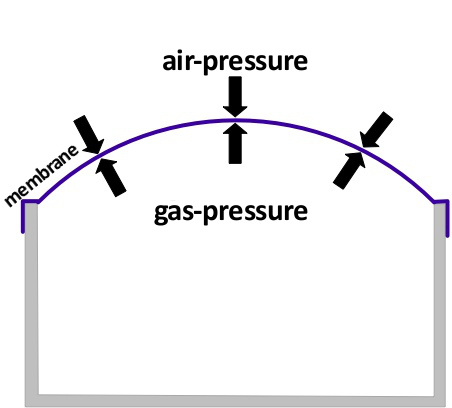
In the picture above the gas inside of the container was heated up. The pressure of a sealed amount of gas increases when the temperature rises. Because the membrane is expendable, it is bulged by the pressure inside of the container. That has the effect that the pressure inside decreases again. The membrane therefore allows the container to have the same pressure as the air outside.
