Atomic structure
All substances are made out of atoms, no matter if they are solid, liquid or gaseous. So several properties of bodies can be traced back to the atomic structure and its interaction.
Although their influence is quite large, atoms are extremely small. If you placed atoms end to end until the chain has a length of one millimetre, you would need 10 millions of atoms.
The atomic structure is of particular importance. An atom consists of a nucleus and a shell. The nucleus consists of positively charged protons and neutrally charged neutrons. The atomic shell consists of electrons which are charged negatively.
It depends on the composition of protons, neutrons and electrons, if the atom forms i.e. iron, gold or oxygen. The following illustration, for example, shows a simple model of an aluminium atom.
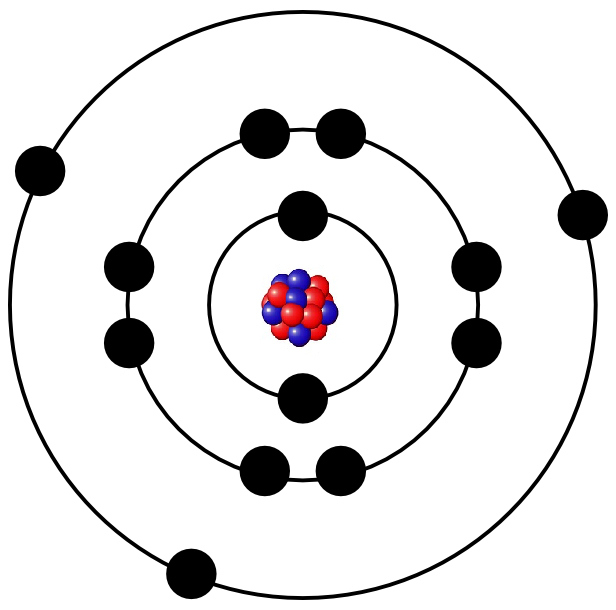
Protons and neutrons are much more heavier than electrons so that almost all of the mass of an atom is bundled in the nucleus. Nevertheless, the electrons form the atomic shell and therefore the majority of the volume of the atom. If the atomic nucleus was as big as the pea, the atomic shell would be as big as the whole stadium with the tribune.
It is of great importance for the electrical science that the charge of objects is defined by their atoms. So usually negative charges, namely electrons, are responsible for the charge transport. These can move more easily because they are not fixed to the nucleus.
