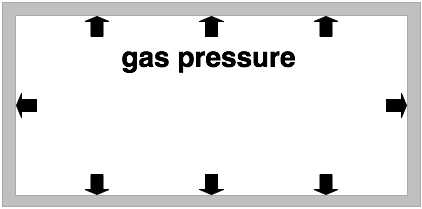
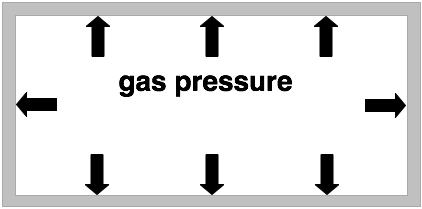
gas pressure
If one has a certain amount of gas inside a container its pressure is proportional to its temperature. (The higher the temperature of a gas the higher its pressure.)
The temperature of a gas is an indicator for the average velocity of the gas particles. The higher the temperature the higher the average velocity of the gas molecules.
In the picture on the left the short arrows indicate the gas pressure at low temperatures. The longer arrows in the picture on the right indicate a higher gas pressure which is a result of the gas being heated.