gas pressure
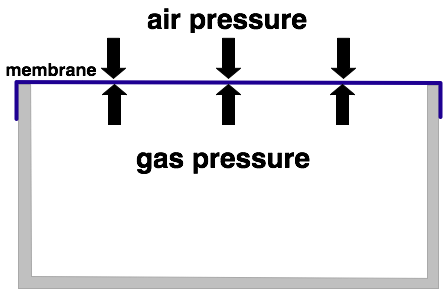
The picture above displays a container that is closed up with a slightly flexible membrane. The pressure of the gas within the container is equal to the external air pressure. Furthermore the temperatures inside and outside are the same.
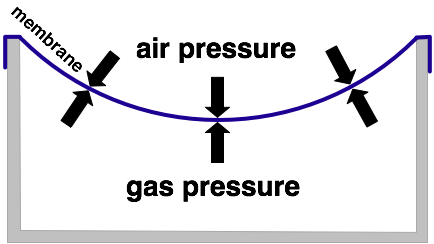
In the picture above the gas has been cooled down. A concealed amount of gas lowers its pressure when temperature decreases. Since the membrane yields the gas inside the container is being compressed by external air pressure causing the internal pressure level to rise.
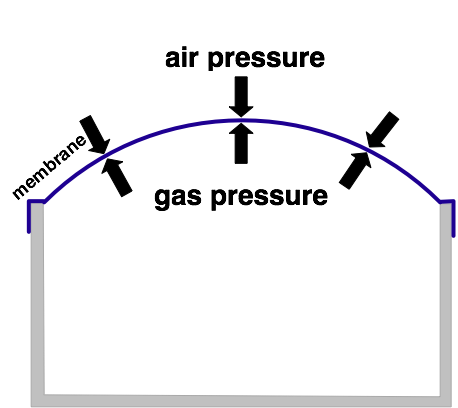
In the picture above the gas has been heated up. A concealed amount of gas increases its pressure when temperature increases. The gas pressure inside the container causes the membrane to bulge out. That is the reason why internal pressure deteriorates again. So the membrane alligns the inside and oustide pressure.
