Temperature and heat
Temperature
The temperature of an object describes a condition at a specific point in time. It measures the inner energy of an object (the term inner energy describes all of the energy of the particles in an object).
When an object is heated up (eg. with a candle) the particles receive additional energy and their movement (or vibration) gets stronges. The inner energy of the object increases. We speak of an increase in temperature.
In Europe, temperatures are measured in degrees Celsius (°C). But there are other units for temperature, such as Kelvin K and degree Fahrenheit °F. Temperature scales differ in their zero point. Celsius picked his zero point as the point at which water freezes. Fahrenheit instead chose the lowest temperature of the bitter winter of the year 1709, which he could reproduce through a mixture in his laboratory. Science uses the Kelvin scale. Kelvin made the absolute zero his lower limit. At this temperature, no particles move.
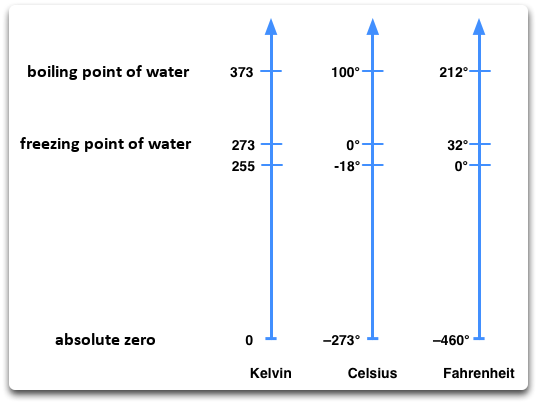
Image: A small overview of the three common temperature scales (Kelvin, Celsius, Fahrenheit).
Heat
Heat is a form of energy. Other forms of energy are for example the mechnical or electrical energy. Heat describes the amount of energy that an object absorbs or emits.
