The first law of thermodynamics
The energy in a closed system does not change. Energy cannot be created from nothing nor can it be destroyed. However, it is possible to transform energy from one form into another (e.g. mechanical energy can be transformed into heat).
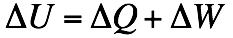
(U = Intermal Energie; Q = Heat; W = Work)
The change of energy of a closed system equals the sum of the change of heat and the change of work. The work that is done on the system and the added heat is supplied with a positive algebraic sign, the performed work and the emitted heat is supplied with a negative algebraic sign.
