Density of substances
Both bodies (left side: wood, right side: aluminium) in the illustration below have a mass of 200 g each. But they very much differ in their size (volume).
If we cut off a piece of wood, which is exactly the size of the piece of aluminium, we would observe that the piece of wood is much lighter than the piece of aluminium.

If we compare substances (for example iron with plastic, ice with copper and so on) in this way, we can summarise this observation in the following way:
Bodies which have the same volume do not have the same mass, if they consist of different materials.
Density describes the relation between the mass of a body and its volume.
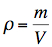
where:
| ρ | density of the substance |
| m | mass of the substance |
| V | volume of the substance |
- The density of solids and fluids is also dependent on the temperature.
- The density of gases is dependent on the temperature as well as the pressure.
A few examples for the density of substances:
| substance | kg/m³ | g/cm³ |
| copper | 8933 | 8,933 |
| aluminium | 2702 | 2,702 |
| gold | 19290 | 19,290 |
| water | 998 | 0,998 |
| ice | 917 | 0,917 |
| air | 1,2929 | 0,0012929 |
| helium | 0,1785 | 0,0001785 |
