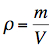Density of substances
|
The two bodies (on the left: wood, on the right: aluminium) in the right figure each have a mass of 200 g. Nevertheless they differ widely in their size (volume). Bodies which are made of different kinds of material do not have the same masses while having the same volume. |

|
|
whereby it applies:
|
Some examples of the density of substances:
|