Solution
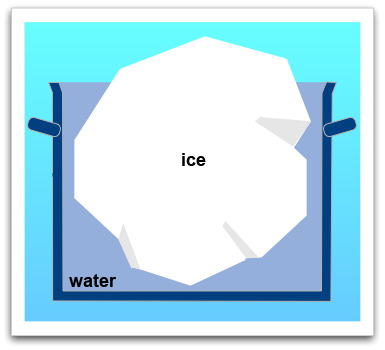
Because the iceberg is swimming on the water the following two points are important:
- The density of the iceberg is smaller than the densitiy of the water because water expands as a result of freezing. This means that ice needs more space than water even though the mass is the same. If the iceberg melts, the generated water will have a smaller volume than the ice had.
- The mass of the water which is displaced by the iceberg equals the mass of the iceberg itself. While melting, the mass of the ice does not change. Therefore the same amount of water must be generated by the melting of the iceberg as was displaced by the iceberg before.
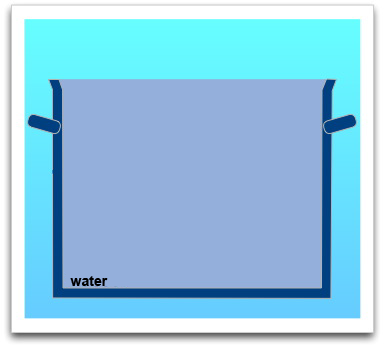
For the pot was filled up to the brim with water when the iceberg was swimming in it, it will still be filled to the brim with water after the iceberg will have melted (picture on the right).
