Inside the battery
In which way electricity is stored in a battery?
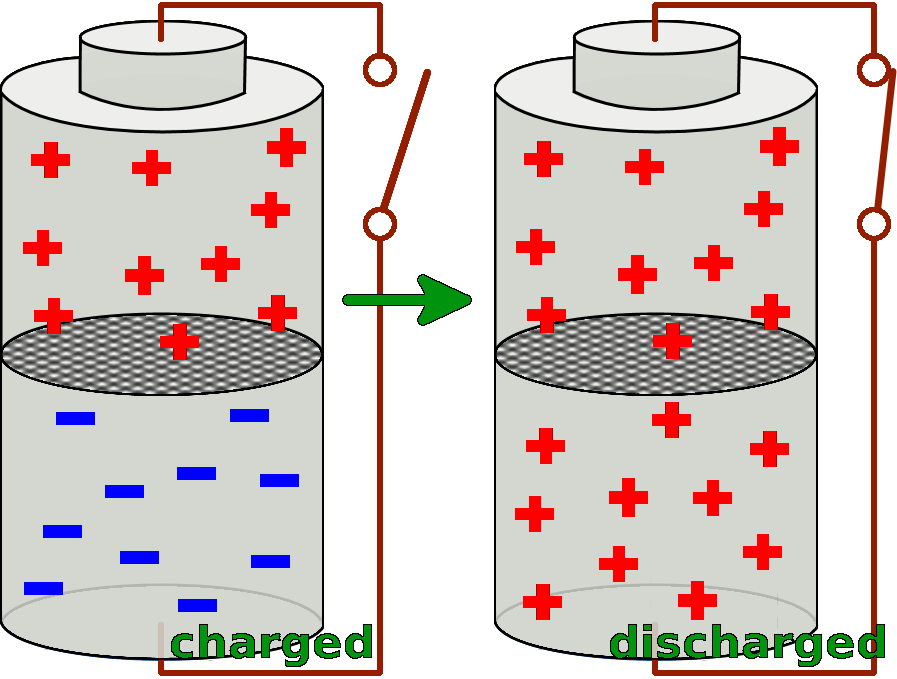
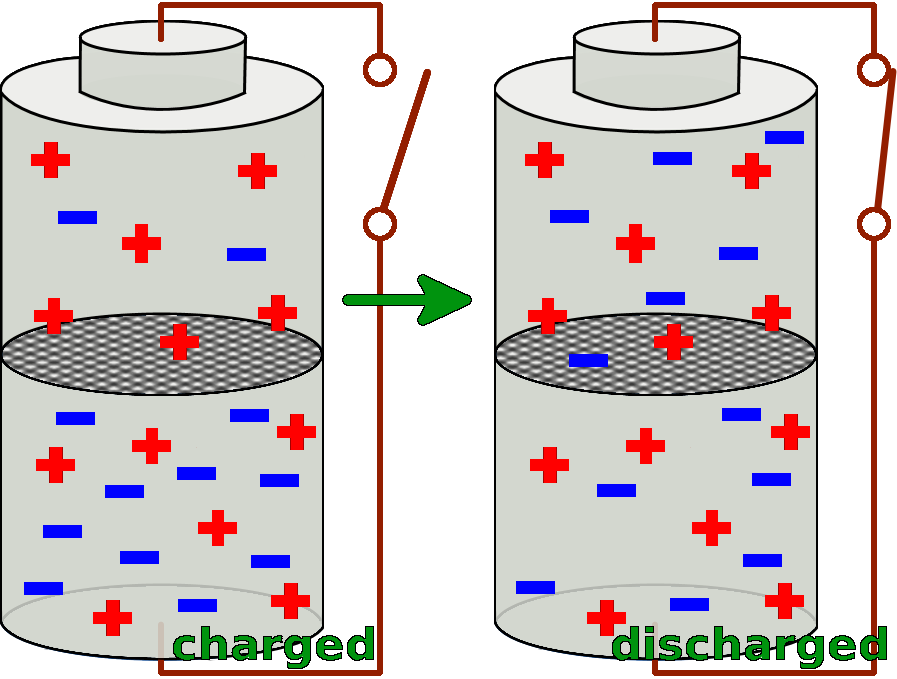
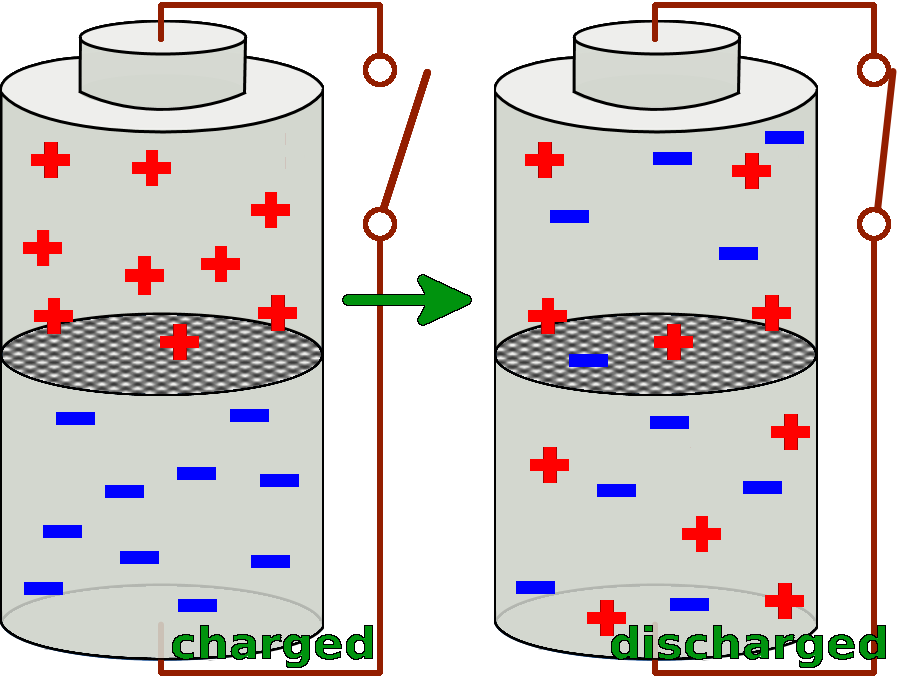
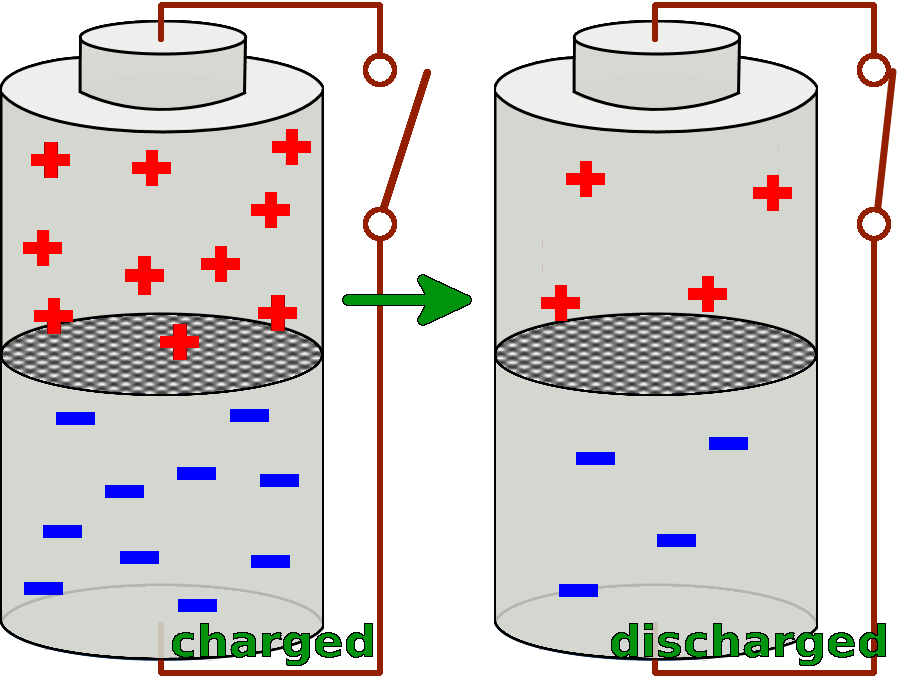
A battery consists of two cells. Both of the two cells are filled with special substances, so that there is a particular charge distribution inside of them. The substance in the lower cell has got a markedly surplus of negative charges, so it delivers these negative charges very easily. Whereas the substance in the upper cell lacks negative electrons and thus takes them up easily.
If now the two cells are connected with a conductor, the different substances can react with each other and exchange negative charges.