Density of gases:
The density of a gas is the relation of mass and volume:
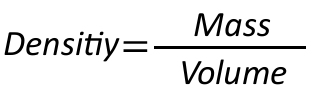
However, this is only true for constant temperatures.
What happens, if a gas is heated up?
|

|
|
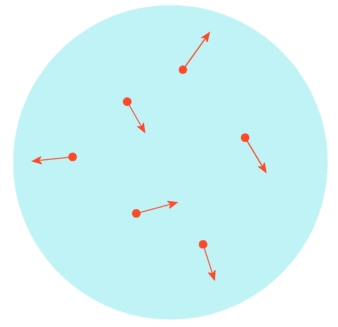
In a cold gas the particles move only a little (short arrows). |
If the gas is heated up the particles move faster and faster (long arrows). The particles need more space because they move faster. Therefore, the gas takes up a bigger volume. |
What happens with the density?
Because the number of particles remained the same, the mass does not change. However, the volume is much bigger now. Therefore, the density must decrease as can be seen in the formula above.
