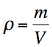Density of materials
|
Both bodies in the picture on the right (on the left: Wood, on the right: Aluminum) have an equal mass of 200 g. Nevertheless they strongly differ in its volume. Bodies with the same volume that are made of different materials differ in their masses. |

|
|
it applies:
|
Here are some examples for the density of materials.
|